Intro: Space travel is fraught with many risks. The loss of retinal function after long duration space flight (LDSF), however, represents one of the more devastating. This phenomenon, presently referred to as, Spaceflight Associated Neuro-ocular Syndrome (SANS), represents the relative uncertainty of the exact etiology and pathogenesis of this condition.[1] This scourge could quite honestly impair the ability of Mankind to explore the cosmos…
“Currently, an unclear, multifaceted mechanism consisting of components of the theory of …cephalad fluid shift, compartmentalization & alternative compartmentalization theories, increased intracranial pressure, differential translaminar pressure gradients, aberrancies in lymphatic drainage systems, intracerebral volume shifts, orbital and cerebral arterial and vortex vein drainage alterations… cyanocobalamin- & folate-dependent one-carbon pathway variances, choroidal volume expansion, and ambient hypercapnic environments onboard the International Space Station ISS may contribute to the SANS constellation.”[2] April, 2021, Ashwini Kini, MD
Wostyn et.al. 2022 postulate that “a recent discovery of an “ocular glymphatic system” can potentially help to unlock mechanisms underlying microgravity-induced optic disc edema. Indeed, a major paradigm shift is currently occurring in our understanding of transport of fluids and solutes through the optic nerve following the recent discovery of an optic nerve glymphatic pathway for influx of cerebrospinal fluid.”[3] Some authors hypothesize that SANS- related compartmentalization of CSF within the orbital SAS, with locally elevated sheath pressures rather than elevated ICP alone may help to explain the optic disc edema observed.[4],[5],[6],[7]
CONCLUSIONS
We contend that “IF” the above purported predisposing physiologies above contribute to a pressure differential on either side of the retina or ocular space and that contributes to the phenomenon of SANS, then our technologies, depicted below, just may be helpful in not only researching these phenomena, but could possibly be employed to mitigate the onset or severity of the condition.
Delta Chase LLC (DeltaChase) invented TWO medical devices representing two separate physiologies (in different stages of development, both of which should modulate the glymphatic circulatory system. Both jugular compression and CO2rebreathing are well delineated in the medical literature and are SAFE and EFFECTIVE, although the FDA has not yet granted claims for using them to manipulate the glymphatic tree. Glymphatic augmentation is thought to occur while sleeping, and although one physiology (mild CO2 rebreathing) has been extensively studied in sleep, neither CO2 rebreathing, nor jugular compression requires sleep to reap the benefits of improving glymphatic circulation (Note: using small amounts of CO2 in sleephas been extensively studied).

The Q-Collar™
The SAGE Recycler™
BACKGROUND & INTRODUCTION
Recently (2013-14), the scientific community contended that The Glymphatic System, including several anatomical spaces and physiologies such as perivascular channels and astrocyte foot processes, act in concert to effectively eliminate waste (proteins and/or metabolites) from the Central Nervous System (CNS). Flow through this glymphatic system aids in the removal of these waste substances and may contribute to quintessential processes involved in normal brain function (such as fluid volume transmission and transport of metabolically required substances). Sleep seems to augment the glymphatic system although supine or prone posture may also augment the system. Notably, aging has been proposed as a primary factor in the slowing of the Glymphatic System’s clearing function, labelled as “clearance,” and it is currently hypothesized to be involved in the etiology of several neurodegenerative diseases, including Alzheimer’s disease.
We propose a conceptual shift employing two innate biologic responses, whether asleep or awake, to augment several crucial aspects of glymphatic system clearance. We contend that modulating an individual’s inspired CO2, separately or in conjunction with jugular compression, will manipulate cerebral venous blood flow (CSF flow) and alter the pH of blood and neural tissues (CO2 only, here) gently opening the blood-brain barrier to at least proteins and sugars. All of which we believe will increase intracranial blood volume/pressure and hydrodynamic flow within the glymphatic tree.
Late in 2009, DeltaChase’s David Smith MD put forth “SLOSH Theory,” to abate Traumatic Brain Injury, after interacting with the neurotrauma team led by Julian Bailes MD (then Chief of Neurosurgery at West Virginia University). The group postulated the use of two well-described, yet not widely known, physiological capabilities of Nature to fill, or “take up,” the compliance of the cranium. Researchers refer to this excess “potential space” as the cranial reserve volume (CRV) of the brain space, and Smith realized that minimizing CRV could mitigate SLOSH-induced energy absorption into the brain. We anticipate that both of these physiologies will also encourage glymphatic FLOW: 1) Jugular Compression, first disclosed and studied in the 1860s, and 2) Carbon dioxide modulation, also used for decades in disease mitigation via CO2 canister delivery and now with a patented, markedly safer mechanism (utilizing the rebreathing of one’s own exhaled breath).
Both serve to fill the compliance of the intracranial space preventing sloshing of ~70% of the liquid brain within its confines. More than 12 years and 25 publications later the Q-Collar™ technology, referenced above, launched commercially (September, 2021) in the USA. Also, at the time of this writing DeltaChase’s SAGE CO2 Recycler Mask™ (which adds non-anatomical dead space in columns for rebreathing) has entered the latter stages of development with a human trial in sleep apnea likely during the month of April 2022. The function of elevated CO2 in the body has been well demonstrated, so that question need not be demonstrated again, however, we are still in process of Proof of Principle human studies to demonstrate that rebreathing one’s own exhalation produces the same effects as specialty cylinder-delivered CO2.
Jugular compression, first mentioned in the medical literature circa 1700,[8] and further elucidated in 1863,[9]was popularized in 1916, and memorialized as the “Queckenstedt Maneuver.” The more than 50-year maneuver remained the “gold standard” prior to commercialization of Head CTs (which came to existence around 1970) to evaluate the patency of the cranio-spinal space. This maneuver has still been utilized, in some fashion, some 100 years thereafter.[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20] Although canvassing the distant historical literature can be challenging, there remains several excellent documents that disclose the physiology as it was studied through the decades. Textbooks of the time (1947) wrote of the mild rise in spinal manometer pressure readings of CSF in observing the net results of jugular compression (as radiographic measures were non-existent in that time era).[21]
Gilland (1966) used the jugular compression test extensively in studying spinal fluid physiology,[22] and it was Gooding et.al. (1984) that discovered that the mere action of turning one’s head contributed to jugular compression.[23]Then, Jayaraman (2012) noted that in a CT angiography study of 108 unselected, consecutive subjects, only 35 (32.4%) had widely patent jugular veins, with the remaining supermajority (67.6%) having some degree of jugular compression.[24] Jugular compression is the norm, not the exception.
This long-tested compression maneuver impedes the jugular venous return, diverting it to the venous capacitance vessels and vertebrals, ultimately increasing the venous blood volume in the cranial cavity and simultaneously reducing the space for cerebrospinal fluid (which then moves caudally), which in turn, increases the intracranial pressure.[25],[26],[27] Although the intracranial pressure is increased by the Q-test in humans, the mean arterial pressure (MAP) does not change.[28] In elasticity of the cranial volume experiments, using Radioactive Iodinated Serum Albumin (RISA), Kitano [1964] found the percentage rise in cranial pool volume was greater with bilateral compression than left or right compression alone (see FIG 1). Intracranial vessels are capable of free expansion during the period of jugular compression.[29] This finding was confirmed recently by our team (Leach et.al.) by producing jugular compression inside an MRI and measuring the statistically significant volume change in the various cerebral venous sinuses (see FIG 2).[30]
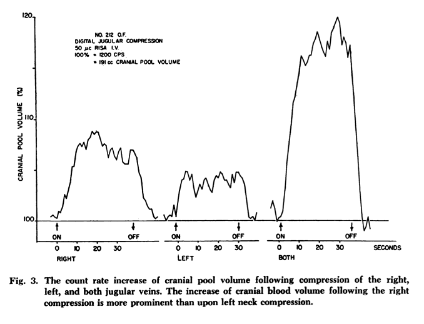
FIG 1.
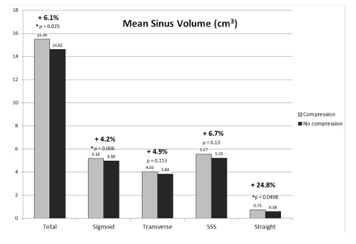
FIG 2.
taken from Kitano et.al. taken from Leach et.al.
Specifically, the physiology of cranio-spinal fluids in the setting of jugular compression has been determined visually by cinemyelographic methods. Introducing 15-18 ml of Pantopaque into the lumbar spinal space under fluoroscopic guidance combined with bilateral jugular compression introduced in the horizontal prone position and contrast dye in the entirety of the spinal column was observed.[31] Gilland concluded that the dynamic fluid events take place in normal patients within a few tenths of a second.[32] He also estimated that the cerebrospinal fluid flow, past the midthoracic level, on bilateral jugular compression was on the order of 2-7 milliliters and in the lumbar region, the main contrast movement noted was a lateral distention of 28-50%.[33]
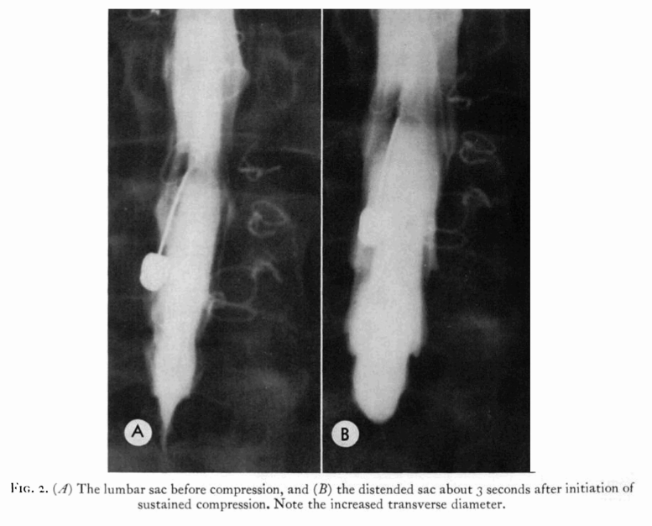 FIG 3
FIG 3
Changes in venous drainage from the cranium, such as reduction in internal jugular venous flow when moving from a supine to upright posture, can alter cerebral hemodynamics and CSF dynamics.[34] In this study, CSF was expelled more rapidly from the cerebral aqueduct with jugular compression.[35]
CO2 EFFECT ON CBF: The SAGE CO2 Recycler MASK™ (gentle CO2 rebreathing)
Based on further inspiration from Nature, our DeltaChase team has been developing a second technology (“fail-safe” CO2rebreathing) that can alter fluid flux flows within the cranium and, specifically, the interaction of venous blood with CSF levels in the cranium and spine. Importantly, the literature reflects multiple demonstrations where tiny amounts of supplemental CO2dramatically and safely eradicate Central and Obstructive Sleep Apnea and sleep disordered breathing,[36],[37],[38],[39] thereby elevating our confidence for the same safety when used on the Glymphatic System. Our SAGE Mask harmlessly utilizes one’s own exhaled breath as the source of CO2 (instead of the expensive and bulky gas canisters). As of April 2022, we will have started human clinical trials with our first-generation prototypes at the Sleep Management Institute of Cincinnati (largest sleep lab in the Ohio/Kentucky/Indiana region).
Mild hypercapnia, unequivocally, raises the middle cerebral arterial flow velocity[40] with a resultant rise in cerebral blood volume, and just as in jugular compression (or a space-occupying lesion), one expects that the CSF or peri-arterial fluid will flow caudally (defining, in part, the physiology of the Glymphatic System). As stated, small amounts of CO2 (just FiCO2 = 0.5% to 1.5%) have been shown to be well tolerated, and nearly unnoticeable, while improving sleep parameters in a number of populations such as Sleep Apnea sufferers[41],[42],[43],[44],[45] and children. Our SAGE Recycler Mask raises the ETCO2 level by roughly 4-6 mmHg (approximately 0.8% FiCO2). In doing so, like the Queckenstedt Maneuver, we fully expect a rise in intracranial blood volume (~4 ml), and a rise in spinal CSF/blood volume of roughly 20 ml; and some purport this “volume effect” could literally define glymphatic flow. This effect could be facilitated during sleep, or anytime where we institute rebreathing (e.g., on a long sortie or in a boring foxhole for the military).
Just as importantly, in settings where a soldier or astronaut might be excited or under stress, release of adrenalin will result in an increase in respiratory rate leading to a hyperventilation (with resultant hypocapnia). The negatives of hypocapnia in this setting are likely too numerous to list in this paper, however, they include: 1) reduced reaction time, 2) loss of spatial awareness, and 3) diminished balance.[46],[47],[48] Using our lightweight, collapsible, SAGE Mask in settings of increased excitement, should mitigate this hypocapnia, resulting in improved functional capabilities of the warfighter. In theory, each use of the Q-Collar or the SAGE Mask, during sleep or awake, supports improved circulation of the glymphatic system.
[1] Stenger M.B., Tarver W.J., Brunstetter T.et al. Risk of Spaceflight Associated Neuro-ocular Syndrome (SANS). NASA Human Research Program Human Health Countermeasures Element https://humanresearchroadmap.nasa.gov/evidence/reports/SANS.pdf NASA Johnson Space Center. 2017; (Available at:) https://humanresearchroadmap.nasa.gov/evidence/reports/SANS.pdf?rnd=0.434276635495143
[2] Lee, A.G., Mader, T.H., Gibson, C.R. et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. npj Microgravity 6, 7 (2020). https://doi.org/10.1038/s41526-020-0097-9
[3] Wostyn P, Gibson CR, Mader TH. The odyssey of the ocular and cerebrospinal fluids during a mission to Mars: the “ocular glymphatic system” under pressure. Eye (Lond). 2022 Apr;36(4):686-691. doi: 10.1038/s41433-021-01721-9. Epub 2021 Aug 9. PMID: 34373611
[4] Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011; 118:2058–69
[5] Lee AG, Tarver WJ, Mader TH, Gibson CR, Hart SF, Otto CA. Neuro-ophthalmology of space flight. J Neuroophthalmological. 2016; 36:85–91.
[6] Mader TH, Gibson CR, Hart SF, Lee AG. Asymmetric papilledema in idiopathic intracranial hypertension. J Neuroophthalmol. 2016; 36:111–2.
[7] Mader TH, Gibson CR, Otto CA, Sargsyan AE, Miller NR, Subramanian PS, Hart SF, Lipsky W, Patel NB, Lee AG. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol. 2017; 37:133–39
[8] John S. Jenkins, The voice of the Castrato, Lancet, 351, 1877-80, 1998
[9] Williams B., Simultaneous cerebral and spinal fluid pressure recordings. 2. Cerebrospinal dissociation with lesions at the foramen magnum. Acta Neurochirurgica 1981; 59:123–42
[10] Gilland O., Chin F., Anderson W.B., Nelson J.R., A cinemyelographic study of cerebrospinal fluid dynamics., Amer. J. Roentgenol. 1969; 106: 369-375
[11] Magnæs, B. Functional Queckenstedt’s test in rheumatoid atlanto-axial luxation. Acta neurochir 38, 89–99 (1977). https://doi.org/10.1007/BF01401545
[12] Jayaraman J.L., Boxerman L.M., Davis R.A., Haas J.M. Rogg, Incidence of Extrinsic Compression of the Internal Jugular Vein in Unselected Patients Undergoing CT Angiography, AJNR Am J Neuroradiol 33:1247–50, August 2012, www.ajnr.org
[13] Frydrychowski AF, Winklewski PJ, Guminski W (2012) Influence of Acute Jugular Vein Compression on the Cerebral Blood Flow Velocity, Pial Artery Pulsation and Width of Subarachnoid Space in Humans. PLoS ONE 7(10): e48245. doi: 10.1371/journal.pone.0048245
[14] Charles A. Gooding, Gary K. Stimac, Jugular Obstruction Caused by Turning of the Head, AJR 142: Feb 1984, 403-406
[15] A. Hatt, S. Cheng, K. Tan, R. Sinkus, and L.E. Bilston, MR Elastography Can Be Used to Measure Brain Stiffness Changes as a Result of Altered Cranial Venous Drainage During Jugular Compression, AJNR Am J Neuroradiol 36:1971–77 Oct 2015 www.ajnr.org
[16] J. TYRRELL, M.B., CH.B., M.R.C.P., OBSERVATIONS ON THE C.S.F. PRESSURE DURING COMPRESSION OF THE JUGULAR Veins ‘A Text book of the Practice of Medicine’, 8th edition, 16lx., (1947)
[17] Chou C-H, Doong M-L, Fuh J-L, Wu J-C, Wang S-J (2013) Queckenstedt’s Test Affects More than Jugular Venous Congestion in Rat. PLoS ONE 8(3): e59409. https://doi.org/10.1371/journal.pone.0059409
[18] J M S Pearce, Queckenstedt’s Manoeuvre, J Neurol Neurosurg Psychiatry 2006 77: 728 doi: 10.1136/jnnp.2005.083618
[19] Stone, Tubridy, Curren, The Effect of Rigid Cervical Collars on Internal Jugular Vein Dimensions, Academy of Emergency Medicine 2010, 17: 100-102, 2009
[20] Stephan MD., The Queckenstedt Test and Lumbar Puncture, Letter to the Journal, Can Med Ass J., June 9, 1962, vol 86, 1079
[21] J. TYRRELL, M.B., CH.B., M.R.C.P., OBSERVATIONS ON THE C.S.F. PRESSURE DURING COMPRESSION OF THE JUGULAR Veins ‘A Text book of the Practice of Medicine’, 8th edition, 16lx., (1947)
[22] Gilland O. Cerebrospinal fluid dynamic diagnosis of spinal block V. Uniform lumbar electromanometrics. Neurology. 1966 Nov;16(11):1110-7. doi: 10.1212/wnl.16.11.1110. PMID: 5950922.
[23] Charles A. Gooding, Gary K. Stimac, Jugular Obstruction Caused by Turning of the Head, AJR 142: Feb 1984, 403-406
[24] Jayaraman J.L., Boxerman L.M., Davis R.A., Haas J.M. Rogg, Incidence of Extrinsic Compression of the Internal Jugular Vein in Unselected Patients Undergoing CT Angiography, AJNR Am J Neuroradiol 33:1247–50, August 2012, www.ajnr.org
[25] Queckenstedt HHG (1916) Zur Diagnose der Rückenmarkskompression. Deutsche Zeitschrift für Nervenheilkunde 55: 325–333.
[26] Haerer AF (1992) Lumbar puncture. In: Dejong’s The Neurologic Examination (Williams and Wilkins, eds), 5th edn, Philadelphia, PA: Lippincott. 763–776
[27] Pearce JM (2006) Queckenstedt’s Manoeuvre. J Neurol Neurosurg Psychiatry 77: 728.
[28] Koster M, Bethlem J (1961) Paroxysmal hypertension and hypotension in patients with spinal cord lesions (poikilopiesis spinalis). Acta Psychiatr Scand 36: 347–368.
[29] Masami Kitano, M.D., William H. Oldendorf, M.D. and Benedict Cassen, Ph.D., The Elasticity of the Cranial Blood Pool, JOURNAL OF NUCLEAR MEDICINE 5:613-625, 1964
[30] James Leach, Gregory Myer, David Smith, Mild Neck Compression Alters Intracranial Venous Sinus Volume: Implications for a Novel Neuroprotective Effect in Concussion, Abstract No: 2762, Cincinnati Children’s Hospital Medical Center
[31] Gilland O., Chin F., Anderson W.B., Nelson J.R., A cinemyelographic study of cerebrospinal fluid dynamics., Amer. J. Roentgenol. 1969; 106: 369-375
[32] Gilland, O, CSF Dynamic Diagnosis of Spinal Block. III: Equation for block influence on cysterno-lumbar electromanometrics. Acta Neurol 1965, 41, suppl. 13, pp 47-74
[33] Gilland, O, CSF Dynamic Diagnosis of Spinal Block. II: Equation for block influence on cysterno-lumbar electromanometrics. Acta Neurol 1965, 41, suppl. 13, pp 487-496
[34] Alperin N, Lee SH, Sivarama Krishnan A, etal. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J Magn Reson Imaging 2005; 22:591–96
[35] A. Hatt, S. Cheng, K. Tan, R. Sinkus, and L.E. Bilston, MR Elastography Can Be Used to Measure Brain Stiffness Changes as a Result of Altered Cranial Venous Drainage During Jugular Compression, AJNR Am J Neuroradiol 36:1971–77 Oct 2015 www.ajnr.org
[36] Robert Joseph Thomas, Robert W. Daly, J. Woodrow Weiss, Low-Concentration Carbon Dioxide is an Effective Adjunct to Positive Airway Pressure in the Treatment of Refractory Mixed Central and Obstructive Sleep- Disordered Breathing, SLEEP, Vol. 28, No. 1, 2005
[37] Xie A, Rankin F, Rutherford R, Bradley TD. Effects of inhaled CO2 and added dead space on idiopathic central sleep apnea. J Appl Physiol (1985). 1997 Mar;82(3):918-26. doi: 10.1152/jappl.1997.82.3.918. PMID: 9074983.
[38] S. Andreas, K. Weidel. G. Hagenah, S. Heindl, Treatment of Cheyne-Stokes respiration with nasal oxygen and carbon dioxide Eur Respir J 1998; 12: 414–419, DOI: 10.1183/09031936.98.12020414 ISSN 0903 – 1936
[39] David S. Patz, M.D., Michael D. Patz, M.D., and Peter H. Hackett, M.D, Dead Space Mask Eliminates Central Apnea at Altitude, HIGH ALTITUDE MEDICINE & BIOLOGY Volume 14, Number 2, 2013, DOI: 10.1089/ham.2012.1111
[40] Kojiro Ide, Michael Eliasziw, and Marc J. Poulin, Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans, J Appl Physiol 95: 129–137, 2003.
[41] Mulchrone, Shokoueinejad and Webster, Topical Review, A review of preventing central sleep apnea by inspired CO2 Physiol. Meas. 37 (2016) R36–R45 doi:10.1088/0967-3334/37/5/R36
[42] Robert Joseph Thomas, Carbon Dioxide in Sleep Medicine: The Next Frontier for Measurement, Manipulation, and Research, Commentary on Wang et al. Hypercapnia is a key correlate of EEG activation and daytime sleepiness in hypercapnic sleep disordered breathing patients. J Clin Sleep Med 2014; 10:517-522.
[43] Rodney D. Steens, Thomas W. Millar, Su Xiaoling, Darren Biberdorf, Patricia Buckle, Mansoor Ahmed and Meir H. Kryger, Effect of Inhaled 3% CO2 on Cheyne-Stokes Respiration in Congestive Heart Failure, Sleep, Vol. 17, No.1, 1994
[44] Xie A, Rankin F, Rutherford R, Bradley TD. Effects of inhaled CO2 and added dead space on idiopathic central sleep apnea. J Appl Physiol (1985). 1997 Mar;82(3):918-26. doi: 10.1152/jappl.1997.82.3.918. PMID: 9074983.
[45] Jerome A. Dempsey, Curtis A. Smith, Tadeuez Przybylowski, Bruno Chenuel, Ailiang Xie, Hideaki Nakayama and James B. Skatrud, The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep, J Physiol 560.1 (2004) pp 1–11 1 Topical Review
[46] JOHN G. LAFFEY, M.D., AND BRIAN P. KAVANAGH, M.B, HYPOCAPNIA, Medical Progress N Engl J Med, Vol. 347, No. 1 · July 4, 2002 · www.nejm.org
[47] Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998 Sep;29(9):1876-81. doi: 10.1161/01.str.29.9.1876. PMID: 9731612.
[48] Vasiliki Sakellari, Adolf0 M. Bronstein, Hyperventilation Effect on Postural Sway, Arch Phys Med Rehabil Vol78, July 1997